 Gene Editing for Good How CRISPR Could Transform Global Development By Bill Gates Today, more people are living healthy, productive lives than ever before. This good news may come as a surprise, but there is plenty of evidence for it. Since the early 1990s, global child mortality has been cut in half. There have been massive reductions in cases of tuberculosis, malaria, and HIV/AIDS. The incidence of polio has decreased by 99 percent, bringing the world to the verge of eradicating a major infectious disease, a feat humanity has accomplished only once before, with smallpox. The proportion of the world’s population in extreme poverty, defined by the World Bank as living on less than $1.90 per day, has fallen from 35 percent to about 11 percent. Continued progress is not inevitable, however, and a great deal of unnecessary suffering and inequity remains. By the end of this year, five million children under the age of five will have died—mostly in poor countries and mostly from preventable causes. Hundreds of millions of other children will continue to suffer needlessly from diseases and malnutrition that can cause lifelong cognitive and physical disabilities. And more than 750 million people—mostly rural farm families in sub-Saharan Africa and South Asia—still live in extreme poverty, according to World Bank estimates. The women and girls among them, in particular, are denied economic opportunity. Some of the remaining suffering can be eased by continuing to fund the development assistance programs and multilateral partnerships that are known to work. These efforts can help sustain progress, especially as the world gets better at using data to help guide the allocation of resources. But ultimately, eliminating the most persistent diseases and causes of poverty will require scientific discovery and technological innovations. That includes CRISPR and other technologies for targeted gene editing. Over the next decade, gene editing could help humanity overcome some of the biggest and most persistent challenges in global health and development. The technology is making it much easier for scientists to discover better diagnostics, treatments, and other tools to fight diseases that still kill and disable millions of people every year, primarily the poor. It is also accelerating research that could help end extreme poverty by enabling millions of farmers in the developing world to grow crops and raise livestock that are more productive, more nutritious, and hardier. New technologies are often met with skepticism. But if the world is to continue the remarkable progress of the past few decades, it is vital that scientists, subject to safety and ethics guidelines, be encouraged to continue taking advantage of such promising tools as CRISPR. FEEDING THE WORLD Earlier this year, I traveled to Scotland, where I met with some extraordinary scientists associated with the Centre for Tropical Livestock Genetics and Health at the University of Edinburgh. I learned about advanced genomic research to help farmers in Africa breed more productive chickens and cows. As the scientists explained, the breeds of dairy cows that can survive in hot, tropical environments tend to produce far less milk than do Holsteins—which fare poorly in hot places but are extremely productive in more moderate climates, thanks in part to naturally occurring mutations that breeders have selected for generations. The scientists in Scotland are collaborating with counterparts in Ethiopia, Kenya, Nigeria, Tanzania, and the United States. They are studying ways to edit the genes of tropical breeds of cattle to give them the same favorable genetic traits that make Holsteins so productive, potentially boosting the tropical breeds’ milk and protein production by as much as 50 percent. Conversely, scientists are also considering editing the genes of Holsteins to produce a sub-breed with a short, sleek coat of hair, which would allow the cattle to tolerate heat. This sort of research is vital, because a cow or a few chickens, goats, or sheep can make a big difference in the lives of the world’s poorest people, three-quarters of whom get their food and income by farming small plots of land. Farmers with livestock can sell eggs or milk to pay for day-to-day expenses. Chickens, in particular, tend to be raised by women, who are more likely than men to use the proceeds to buy household necessities. Livestock help farmers’ families get the nutrition they need, setting children up for healthy growth and success in school. Similarly, improving the productivity of crops is fundamental to ending extreme poverty. Sixty percent of people in sub-Saharan Africa earn their living by working the land. But given the region’s generally low agricultural productivity—yields of basic cereals are five times higher in North America—Africa remains a net importer of food. This gap between supply and demand will only grow as the number of mouths to feed increases. Africa’s population is expected to more than double by 2050, reaching 2.5 billion, and its food production will need to match that growth to feed everyone on the continent. The challenge will become even more difficult as climate change threatens the livelihoods of smallholder farmers in Africa and South Asia. Gene editing to make crops more abundant and resilient could be a lifesaver on a massive scale. The technology is already beginning to show results, attracting public and private investment, and for good reason. Scientists are developing crops with traits that enhance their growth, reduce the need for fertilizers and pesticides, boost their nutritional value, and make the plants hardier during droughts and hot spells. Already, many crops that have been improved by gene editing are being developed and tested in the field, including mushrooms with longer shelf lives, potatoes low in acrylamide (a potential carcinogen), and soybeans that produce healthier oil. Improving the productivity of crops is fundamental to ending extreme poverty. For a decade, the Bill & Melinda Gates Foundation has been backing research into the use of gene editing in agriculture. In one of the first projects we funded, scientists from the University of Oxford are developing improved varieties of rice, including one called C4 rice. Using gene editing and other tools, the Oxford scientists were able to rearrange the cellular structures in rice plant leaves, making C4 rice a remarkable 20 percent more efficient at photosynthesis, the process by which plants convert sunlight into food. The result is a crop that not only produces higher yields but also needs less water. That’s good for food security, farmers’ livelihoods, and the environment, and it will also help smallholder farmers adapt to climate change. Such alterations of the genomes of plants and even animals are not new. Humans have been doing this for thousands of years through selective breeding. Scientists began recombining DNA molecules in the early 1970s, and today, genetic engineering is widely used in agriculture and in medicine, the latter to mass-produce human insulin, hormones, vaccines, and many drugs. Gene editing is different in that it does not produce transgenic plants or animals—meaning it does not involve combining DNA from different organisms. With CRISPR, enzymes are used to target and delete a section of DNA or alter it in other ways that result in favorable or useful traits. Most important, it makes the discovery and development of innovations much faster and more precise. PAULO WHITAKER / REUTERS Genetically modified mosquitos, Brazil, ENDING MALARIA In global health, one of the most promising near-term uses of gene editing involves research on malaria. Although insecticide-treated bed nets and more effective drugs have cut malaria deaths dramatically in recent decades, the parasitic disease still takes a terrible toll. Every year, about 200 million cases of malaria are recorded, and some 450,000 people die from it, about 70 percent of them children under five. Children who survive often suffer lasting mental and physical impairments. In adults, the high fever, chills, and anemia caused by malaria can keep people from working and trap families in a cycle of illness and poverty. Beyond the human suffering, the economic costs are staggering. In sub-Saharan Africa, which is home to 90 percent of all malaria cases, the direct and indirect costs associated with the disease add up to an estimated 1.3 percent of GDP—a significant drag on countries working to lift themselves out of poverty. With sufficient funding and smart interventions using existing approaches, malaria is largely preventable and treatable—but not completely. Current tools for prevention, such as spraying for insects and their larvae, have only a temporary effect. The standard treatment for malaria today—medicine derived from artemisinin, a compound isolated from an herb used in traditional Chinese medicine—may relieve symptoms, but it may also leave behind in the human body a form of the malaria parasite that can still be spread by mosquitoes. To make matters worse, the malaria parasite has begun to develop resistance to drugs, and mosquitoes are developing resistance to insecticides. Efforts against malaria must continue to make use of existing tools, but moving toward eradication will require scientific and technological advances in multiple areas. For instance, sophisticated geospatial surveillance systems, combined with computational modeling and simulation, will make it possible to tailor antimalarial efforts to unique local conditions. Gene editing can play a big role, too. There are more than 3,500 known mosquito species worldwide, but just a handful of them are any good at transmitting malaria parasites between people. Only female mosquitoes can spread malaria, and so researchers have used CRISPR to successfully create gene drives—making inheritable edits to their genes—that cause females to become sterile or skew them toward producing mostly male offspring. Scientists are also exploring other ways to use CRISPR to inhibit mosquitoes’ ability to transmit malaria—for example, by introducing genes that could eliminate the parasites as they pass through a mosquito’s gut on their way to its salivary glands, the main path through which infections are transmitted to humans. In comparable ways, the tool also holds promise for fighting other diseases carried by mosquitoes, such as dengue fever and the Zika virus. It will be several years, however, before any genetically edited mosquitoes are released into the wild for field trials. Although many questions about safety and efficacy will have to be answered first, there is reason to be optimistic that creating gene drives in malaria-spreading mosquitoes will not do much, if any, harm to the environment. That’s because the edits would target only the few species that tend to transmit the disease. And although natural selection will eventually produce mosquitoes that are resistant to any gene drives released into the wild, part of the value of CRISPR is that it expedites the development of new approaches—meaning that scientists can stay one step ahead. THE PATH FORWARD Like other new and potentially powerful technologies, gene editing raises legitimate questions and understandable concerns about possible risks and misuse. How, then, should the technology be regulated? Rules developed decades ago for other forms of genetic engineering do not necessarily fit. Noting that gene-edited organisms are not transgenic, the U.S. Department of Agriculture has reasonably concluded that genetically edited plants are like plants with naturally occurring mutations and thus are not subject to special regulations and raise no special safety concerns. The benefits of emerging technologies should not be reserved only for people in developed countries. Gene editing in animals or even humans raises more complicated questions of safety and ethics. In 2014, the World Health Organization issued guidelines for testing genetically modified mosquitoes, including standards for efficacy, biosafety, bioethics, and public participation. In 2016, the National Academy of Sciences built on the WHO’s guidelines with recommendations for responsible conduct in gene-drive research on animals. (The Gates Foundation co-funded this work with the National Institutes of Health, the Foundation for the National Institutes of Health, and the Defense Advanced Research Projects Agency.) These recommendations emphasized the need for thorough research in the lab, including interim evaluations at set points, before scientists move to field trials. They also urged scientists to assess any ecological risks and to actively involve the public, especially in the communities and countries directly affected by the research. Wherever gene-editing research takes place, it should involve all the key stakeholders—scientists, civil society, government leaders, and local communities—from wherever it is likely to be deployed. Part of the challenge in regulating gene editing is that the rules and practices in different countries may differ widely. A more harmonized policy environment would prove more efficient, and it would probably also raise overall standards. International organizations, especially of scientists, could help establish global norms. Meanwhile, funders of gene-editing research must ensure that it is conducted in compliance with standards such as those advanced by the WHO and the National Academy of Sciences, no matter where the research takes place. When it comes to gene-editing research on malaria, the Gates Foundation has joined with others to help universities and other institutions in the regions affected by the disease to conduct risk assessments and advise regional bodies on experiments and future field tests. The goal is to empower affected countries and communities to take the lead in the research, evaluate its costs and benefits, and make informed decisions about whether and when to apply the resulting technology. Finally, it’s important to recognize the costs and risks of failing to explore the use of new tools such as CRISPR for global health and development. The benefits of emerging technologies should not be reserved only for people in developed countries. Nor should decisions about whether to take advantage of them. Used responsibly, gene editing holds the potential to save millions of lives and empower millions of people to lift themselves out of poverty. It would be a tragedy to pass up the opportunity. Full PDF Here
0 Comments
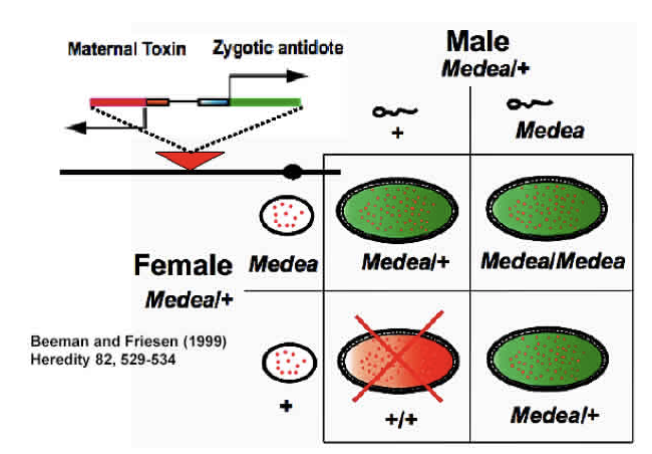
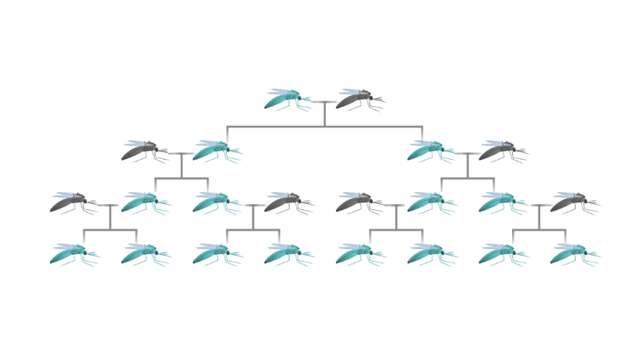 GENE DRIVES AND THE POTENTIAL BENEFITS OF CRISPR TECHNOLOGY APRIL 9, 2018 BY MATTHEW EDGINGTON Marshall and Akbari review a range of different proposed gene drive systems and discuss ways in which CRISPR may be useful in engineering them in a recent issue of ACS Chemical Biology. In the years since translocations were first suggested as a genetics-based method for the control of insect populations, a number of different gene drive strategies have been proposed. To date, progress toward fully functioning versions of each of these systems has been extremely varied. As such, the rapid advancement of CRISPR gene editing technology has given hope that the development of a wide range of gene drive systems should be simplified and accelerated. A SELFISH GENE (BLUE) SPREADING THROUGH A MOSQUITO POPULATION This review is organised according to the expected behaviour of the systems discussed. In particular, the authors discuss threshold-dependent drives (translocations and engineered underdominance), threshold-independent drives (Medea, homing-based systems and driving Y chromosomes) and temporally self-limiting drives (killer-rescue and daisy drives). For each system discussed, the authors outline key details including the drive mechanism, predicted dynamics following release into a wild population and current progress toward engineering them. For the non-CRISPR-based systems discussed here (i.e. translocations, engineered underdominance, Medeaand killer-rescue), a number of ways in which CRISPR technology could accelerate gene drive development are proposed. Specifically, the authors note that CRISPR should provide a new means of engineering lethal toxins and also that CRISPR has already been used to generate site-specific chromosomal translocations. THE TOXIN-ANTIDOTE – BASED DRIVE SYSTEM KNOWN AS MEDEA. EMBRYOS WITHOUT MEDEA WILL DIE BECAUSE OF MATERNALLY DEPOSITED ‘TOXINS’. While discussing threshold-independent drive systems, the authors point out the need to develop remediation measures in case such a system were to produce unintended/undesirable consequences. As such, for Medeathey discuss the possibility of releasing a second generation element that should spread at the expense of both the original version and the wild-type allele. For homing-based systems a range of different remediation strategies are discussed, namely ERACR, CHACR and an immunizing reversal drive. As these are summarized in the review, we do not outline their workings here. AN EXAMPLE OF AN ENGINEERED UNDERDOMINANCE SYSTEM Finally, the authors discuss the recently proposed (and not yet developed or extensively modelled) daisy quorum drive system. Briefly, this would use either a daisy-chain or daisyfield drive system to produce an underdominance effect in a target population. Thus, daisy quorum is proposed as a method for generating threshold-dependence using a temporally self-limiting drive system. This paper provides a good review of a range of different gene drive strategies, some challenges encountered in engineering them and opportunities whereby CRISPR technology could help simplify/accelerate the development of these systems. John M. Marshall and Omar S. Akbari (2018) Can CRISPR-Based Gene Drive Be Confined in the Wild? A Question for Molecular and Population Biology. ACS Chem. Biol. 13. 424-430 https://pubs.acs.org/doi/abs/10.1021/acschembio.7b00923 |
Archives
June 2024
|

 RSS Feed
RSS Feed
